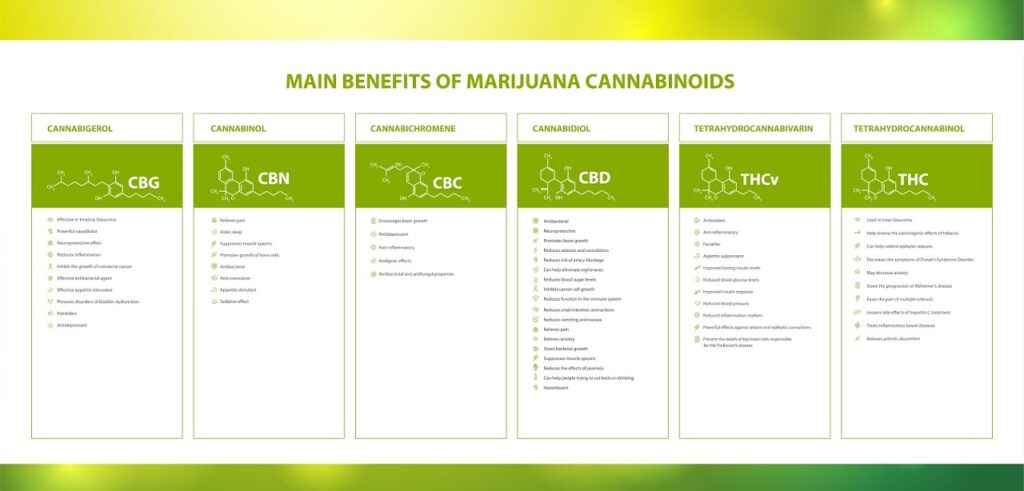
Over 100 unique cannabinoids have been identified in cannabis plants, with tetrahydrocannabinol (THC or ∆9-THC) and cannabidiol (CBD) being the most prominent and well-researched compounds1,2.
Cannabinoids are produced via biosynthesis. This is the process in which enzymes cause a series of chemical reactions that create complex molecules out of simple ones7.
Other common cannabinoids include:2
- Delta 8-tetrahydrocannabinol (∆8-THC)
- Cannabigerol (CBG)
- Cannabinol (CBN)
- Cannabichromene (CBC)
- Cannabivarin (CBV)
- Cannabidivarin (CBDV)
- Delta 9-tetrahydrocannabivarin (THCV)
The most recently discovered cannabinoids are41,42,97,98,103:
- Delta-9 Tetrahydrocannabiphorol (∆9-THCP)
- Cannabidiphorol (CBDP)
- Cannabimovone (CBM)
Cannabinoids v.s. Phytocannabinoids
While you may see these terms used interchangeable, they do have technical differences3.
Cannabinoid is a broad term for compounds produced by plants and mammals alike. These compounds help balance and regulate a variety of biological functions.
Phytocannabinoids are produced by plants, whereas endocannabinoids are produced endogenously (internally, naturally) by mammals3,8.
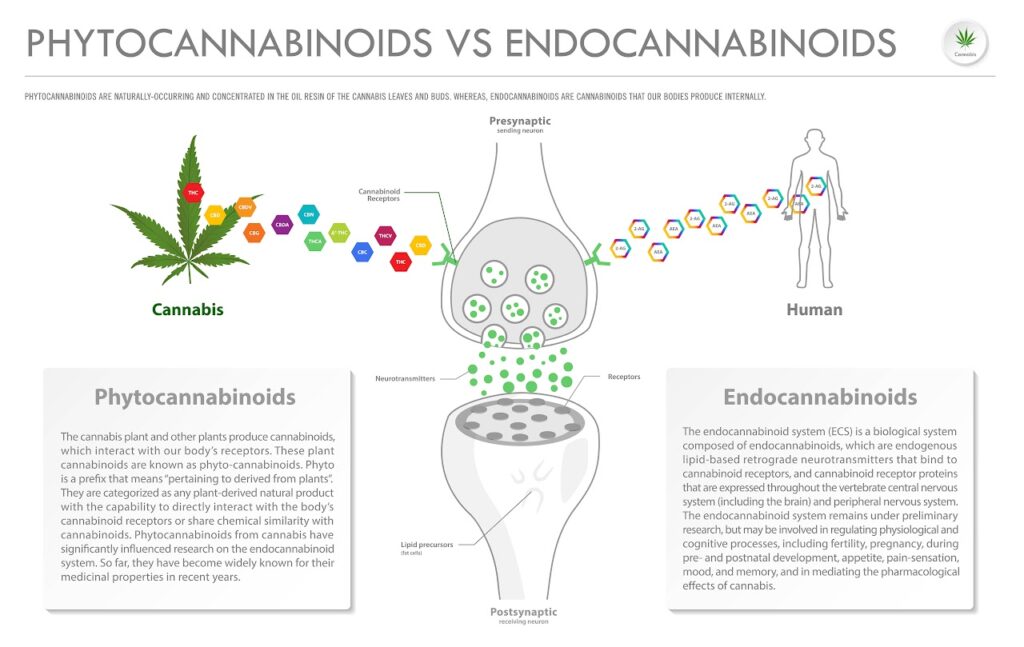
“Endogenous cannabinoids, or endocannabinoids, are cannabinoids produced inside the mammalian body. Every function in our bodies requires a specific balance of factors in order to perform at maximum capacity. When this balance is achieved, it’s called homeostasis. Endocannabinoids play a major role in survival by helping the body maintain homeostasis. Because our bodies already use cannabinoid molecules to regulate many functions, we’re inherently endowed with many targets the cannabis plant can activate.”3
Despite these technical differences, phytocannabinoids and endocannabinoids aren’t structurally different from each other3. They both activate the endocannabinoid system, but it appears endocannabinoids have a homeostatic effect, whereas phytocannabinoids produce medicinal effects.
However, unlike endocannabinoids, phytocannabinoids “cannot be degraded by our body”20. Instead, biotransformation occurs in the liver to trigger a series of chemical events (hydroxylation and oxidation) until phytocannabinoids are broken down into a water-soluble compound (glucuronide) that can be easily excreted.
Lastly, phytocannabinoids aren’t exclusive to cannabis — they’re found in other plant species, as well8. Phytocannabinoids found in other plants also interact with the endocannabinoid system.
Endocannabinoids
The two most studied endocannabinoids are N-arachidonoylethanolamine (anandamide) and 2-arachidonoylglycerol (2-AG)4,8,20.
However, endocannabinoids and phytocannabinoids target receptors outside the endocannabinoid system, as well4.
Cannabinoids share pathways and enzymes with “‘endocannabinoid-like’ mediators,” which may or may not interact with the same proteins as cannabinoids4,20.
Endocannabinoid-like mediators are mediators that share the same chemical class as endocannabinoids (amides or esters of long-chain fatty acids), and the same degrading pathways as well as enzymes4.
Endocannabinoid-like mediators differ from anandamide and 2-AG, as their preferred protein receptors aren’t CB1 and CB2. Rather, it’s thought endocannabinoid-like mediators indirectly influence endocannabinoid levels or their actions4. “The endocannabinoid-like compounds do not bind to CB1 nor to CB2, but inhibit endocannabinoid degradation thus prolonging their biological activity with an ‘entourage effect.’”20
The Entourage Effect
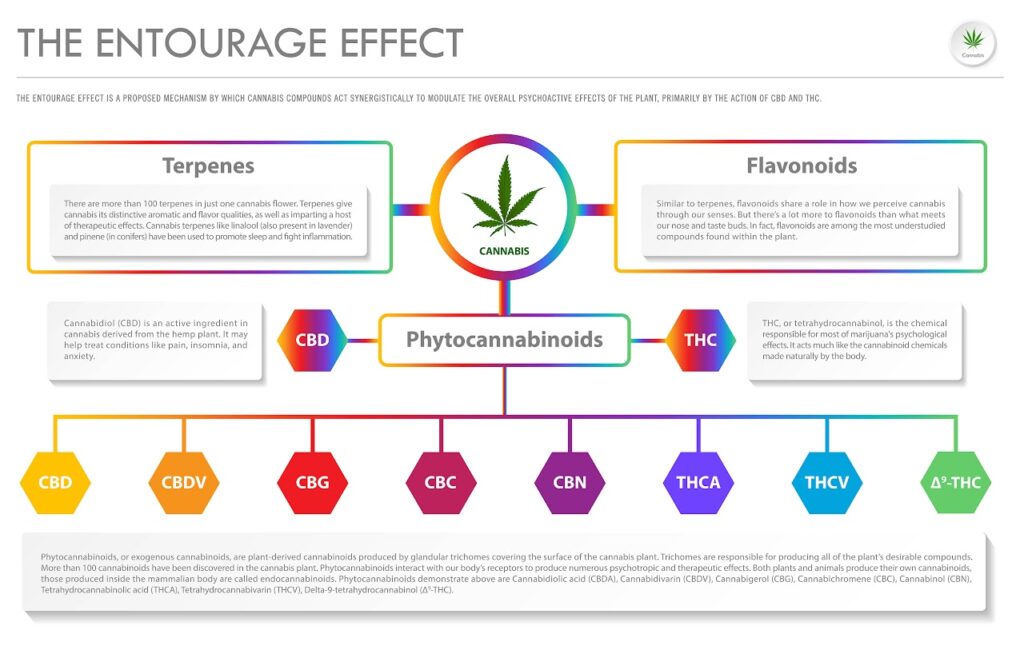
The entourage effect is a popular (and widely accepted) theory that all of these compounds — namely phytocannabinoids and terpenes — work together to produce unique effects21. This would explain why the effects of cannabis products can vary greatly, even within the same strain or from the same plant21,20
Cannabinoid Acids (Raw Cannabinoids — THCa, CBDa, CBGa)
The “acid” form of phytocannabinoids (THCa, CBDa, CBGa, etc…) are the “pre-cannabinoids” produced by the cannabis plant’s cannabinoid biosynthetic pathways22,23. These compounds become THC, CBD, and CBG through oxidation (aging) and decarboxylation (heating) due to the oxidative instability of alkylic cannabinoids22.
In other words, cannabinoid acids (for example, THCa) are raw cannabinoids, whereas the non-acid forms are the aged and heated cannabinoids. THCa and CBDa are sometimes isolated in concentrated forms to create “Crystalline THC or CBD,” the “purest form of [cannabis] concentrate available”24. Additionally, THCa and CBDa have demonstrated similar anti-nausea, anti-inflammatory, and anti-tumor properties as their heated and aged forms, THC and CBD43.
CBGa is unique in that it is found in high concentrations in the cannabis plant during its developmental stages2,23. As the plant matures, CBGA is converted into THCa and CBDa. Any CBGa that remains will become CBG through decarboxylation23.
Synthetic Cannabinoids
“Synthetic cannabinoids are not one drug. Hundreds of different synthetic cannabinoid chemicals are manufactured and sold. New ones with unknown health risks become available each year. Synthetic cannabinoids are popular because users often believe they are legal and relatively safe.”17
By design, synthetic cannabinoids are created to mimic THC and other phytocannabinoids17,18. Initially, they were created to help researchers further explore the endocannabinoid system, but have quickly spread to the point of being abused worldwide18.
However, many of these synthetic chemicals are quite different from phytocannabinoids, making them unpredictable and even deadly in some cases17,19.
“K2,” “Spice,” and “Kronic” are a few examples of “brand name” synthetic cannabinoids, according to the CDC17.
Contrary to popular misconceptions, many synthetic cannabinoids are illegal. They can also cause a variety of negative side effects, including:
- Agitation and irritability
- Confusion and concentration difficulties
- Delusions, hallucinations
- Difficulty breathing
- Gastrointestinal distress
- Heart attack
- Kidney failure
- Muscle damage
- Seizures
- Sleepiness and dizziness
- Death
Synthetic cannabinoids may also be addictive, which can lead to severe withdrawal symptoms upon abruptly ceasing use of them.
Kay Takeaways
Over 100 unique phytocannabinoids have been identified in cannabis plants, with tetrahydrocannabinol (THC or ∆9-THC) and cannabidiol (CBD) being the most prominent and well-researched compounds1,2. Other common phytocannabinoids include Delta 8-THC, CBG, CBN, CBC, CBV, CBDV, and THCV.
While the terms “cannabinoids” and “phytocannabinoids” are often used interchangeably, there are technical terms between them. Cannabinoid is a broad term for compounds produced by plants and mammals alike. These compounds help balance and regulate a variety of biological functions. Phytocannabinoids are produced by plants, whereas endocannabinoids are produced endogenously (internally, naturally) by mammals3,8.
In other words, all phytocannabinoids and endocannabinoids are cannabinoids, but not all cannabinoids are endocannabinoids or phytocannabinoids (they’re either one or the other).
Therapeutic benefits have been identified for all phytocannabinoids, with some producing pain-relieving effects, inhibiting inflammation, reducing anxiety, improving sleep, and decreasing nausea. Some phytocannabinoids also have neuroprotective, anticonvulsant, and antioxidant properties2. Likewise, some cannabinoids are psychoactive while others (namely, derivatives of CBD) are non-psychoactive.
Considering that over 100 unique phytocannabinoids have been identified in cannabis plants, it’s interesting to ponder how research on cannabinoids will progress in the years to come1,2. Perhaps the list of common cannabinoids will grow to include even more compounds.
However, there’s still plenty to learn about the cannabinoids we’ve already focused on over the last few decades.
For example, CBD products represent a $1.6 billion industry in the United States97, but its role in the endocannabinoid system remains elusive2,7. Additionally, very little research has been done on the exact pharmacology of CBV2,35.
Continuing to expand our understanding of cannabinoids is critical to appreciating the entourage effect, which will enhance our ability to recommend specific strains for achieving specific medicinal benefits.
References
- Spindle, T. R., Bonn-Miller, M. O., & Vandrey, R. (2019). Changing landscape of cannabis: novel products, formulations, and methods of administration. Current Opinion in Psychology, 30, 98–102. https://doi.org/10.1016/j.copsyc.2019.04.002
- Morales, P., Hurst, D. P., & Reggio, P. H. (2017). Molecular Targets of the Phytocannabinoids: A Complex Picture. Progress in the Chemistry of Organic Natural Products, 103–131. https://doi.org/10.1007/978-3-319-45541-9_4
- WeedMaps. (n.d.). What is a Phytocannabinoid? | Phytocannabinoid Definition by Weedmaps. https://weedmaps.com/learn/dictionary/phytocannabinoid/
- Di Marzo, V., & Piscitelli, F. (2015). The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics, 12(4), 692–698. https://doi.org/10.1007/s13311-015-0374-6
- About time. (n.d.-b). Human CBD Receptor Chart [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-cbd-receptor-chart-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/267901368?asset_id=267901368
- About time. (n.d.-c). Human Endocannabinoid System [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-endocannabinoid-system-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/276860924?prev_url=detail
- Raypole, C., & Carter, A. (2019, May 17). A Simple Guide to the Endocannabinoid System. Healthline. https://www.healthline.com/health/endocannabinoid-system
- Silver, R. J. (2019). The Endocannabinoid System of Animals. Animals, 9(9), 686. https://doi.org/10.3390/ani9090686
- Muller, C., Morales, P., & Reggio, P. H. (2019). Cannabinoid Ligands Targeting TRP Channels. Frontiers in Molecular Neuroscience, 11, 0. https://doi.org/10.3389/fnmol.2018.00487
- Ahimsadasan, N. (2021, January 13). Neuroanatomy, Dorsal Root Ganglion – StatPearls – NCBI Bookshelf. NCBI. https://www.ncbi.nlm.nih.gov/books/NBK532291/
- marina_ua. (n.d.). The nervous system [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-nervous-system/94711659?prev_url=detail
- The Editors of Encyclopaedia Britannica. (2014, January). Osteoclast | cell. Encyclopedia Britannica. https://www.britannica.com/science/osteoclast
- Akopian, A. N., Ruparel, N. B., Jeske, N. A., Patwardhan, A., & Hargreaves, K. M. (2009). Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends in Pharmacological Sciences, 30(2), 79–84. https://doi.org/10.1016/j.tips.2008.10.008
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants, 9(1), 21. https://doi.org/10.3390/antiox9010021
- Pellati, F., Borgonetti, V., Brighenti, V., Biagi, M., Benvenuti, S., & Corsi, L. (2018). Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Research International, 2018, 1–15. https://doi.org/10.1155/2018/1691428
- Marrone, M. C., Morabito, A., Giustizieri, M., Chiurchiù, V., Leuti, A., Mattioli, M., Marinelli, S., Riganti, L., Lombardi, M., Murana, E., Totaro, A., Piomelli, D., Ragozzino, D., Oddi, S., Maccarrone, M., Verderio, C., & Marinelli, S. (2017). TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nature Communications, 8(1), 0. https://doi.org/10.1038/ncomms15292
- Centers For Disease Control and Prevention. (2017, August 21). About synthetic cannabinoids. https://www.cdc.gov/nceh/hsb/chemicals/sc/About.html
- Wiley, J., Marusich, J., Huffman, J. W., Balster, R. L., & Thomas, B. (2011). Hijacking of Basic Research: The Case of Synthetic Cannabinoids. RTI Press, 0. https://doi.org/10.3768/rtipress.2011.op.0007.1111
- Peñaloza, M. (2018, July 27). NPR Cookie Consent and Choices. NPR. https://choice.npr.org/index.html?origin=https://www.npr.org/2018/07/27/632261920/d-c-has-had-more-than-300-suspected-k2-overdoses-in-2-weeks
- Maccarrone, M. (2020). Phytocannabinoids and endocannabinoids: different in nature. Rendiconti Lincei. Scienze Fisiche e Naturali, 31(4), 931–938. https://doi.org/10.1007/s12210-020-00957-z
- Rahn, B. (2020, September 29). The entourage effect: How cannabis compounds may be working together. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-entourage-effect-why-thc-and-cbd-only-medicines-arent-g
- Gülck, T., & Møller, B. L. (2020). Phytocannabinoids: Origins and Biosynthesis. Trends in Plant Science, 25(10), 985–1004. https://doi.org/10.1016/j.tplants.2020.05.005
- Beadle, A. (2020, July 27). CBG vs CBD: What Are the Differences? Analytical Cannabis. https://www.analyticalcannabis.com/articles/cbg-vs-cbd-what-are-the-differences-312232
- Bennett, P. (2020a, July 28). THCA and CBD Crystalline: Cannabinoids at Their Purest. Leafly. https://www.leafly.com/news/strains-products/what-are-thca-cbda-crystalline-cannabinoids
- Morano, A., Fanella, M., Albini, M., Cifelli, P., Palma, E., Giallonardo, A. T., & Di Bonaventura, C. (2020). Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatric Disease and Treatment, Volume 16, 381–396. https://doi.org/10.2147/ndt.s203782
- Scaccia, A., & Wilson, D. (2020, August 19). Serotonin: What You Need to Know. Healthline. https://www.healthline.com/health/mental-health/serotonin
- Ameri, A. (1999). The effects of cannabinoids on the brain. Progress in Neurobiology, 58(4), 315–348. https://doi.org/10.1016/s0301-0082(98)00087-2
- Lucas, C. J., Galettis, P., & Schneider, J. (2018, July 12). The pharmacokinetics and the pharmacodynamics of cannabinoids. British Journal of Clinical Pharmacology. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.13710
- National Cancer Institute. (n.d.). NCI Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/delta-8-tetrahydrocannabinol?redirect=true
- Shannon, S. (2019). Cannabidiol in Anxiety and Sleep: A Large Case Series. The Permanente Journal, 23, 0. https://doi.org/10.7812/tpp/18-041
- Grandy, D. (2016). Amphetamines Activate G-Protein Coupled Trace Amine-Associated Receptor 1 (TAAR1). In Neuropathology of drug addictions and substance misuse. (pp. 108–116). Academic Press,. https://doi.org/10.1016/B978-0-12-800212-4.00010-8
- Raypole, C., & Sullivan, D. (2020, March 30). Cannabis Got You Paranoid? How to Deal With It. Healthline. https://www.healthline.com/health/marijuana-paranoia
- Walters, O., & Kerr, M. (2020, October 7). A Straightforward Cannabinoid Chart With Simple Explanations. Sovereignty. https://sovereignty.co/cannabinoid-chart/
- S. (2020, July 15). The Terpenes Of Cannabis, Their Aromas, And Effects. THCFarmer – Cannabis Cultivation Network. https://www.thcfarmer.com/threads/the-terpenes-of-cannabis-their-aromas-and-effects.68222/
- Marijuana Doctors. (2018, November 20). Cannabivarin (CBV) | What Is CBV. https://www.marijuanadoctors.com/resources/cannabinoids/cannabivarin-cbv/
- Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., Heasman, M., Edden, R. A. E., Williams, S., Murphy, D. G. M., Daly, E., & McAlonan, G. M. (2019). Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): a single dose trial during magnetic resonance spectroscopy. Translational Psychiatry, 9(1), 0. https://doi.org/10.1038/s41398-019-0654-8
- Abioye, A., Ayodele, O., Marinkovic, A., Patidar, R., Akinwekomi, A., & Sanyaolu, A. (2020). Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. Journal of Cannabis Research, 2(1), 0. https://doi.org/10.1186/s42238-020-0016-7
- García, C., Palomo-Garo, C., García-Arencibia, M., Ramos, J. A., Pertwee, R. G., & Fernández-Ruiz, J. (2011). Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. British Journal of Pharmacology, 163(7), 1495–1506. https://doi.org/10.1111/j.1476-5381.2011.01278.x
- Seladi-Schulman, J., & Kay, C. (2019, August 19). In Vivo vs. In Vitro: What Does It All Mean? Healthline. https://www.healthline.com/health/in-vivo-vs-in-vitro
- Coombs, D. W. & The Journal of the American Medical Association. (1983, November 4). antinociception. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/medical/antinociception
- Citti, C., Linciano, P., Russo, F., Luongo, L., Iannotta, M., Maione, S., Laganà, A., Capriotti, A. L., Forni, F., Vandelli, M. A., Gigli, G., & Cannazza, G. (2019). A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Scientific Reports, 9(1), 0. https://doi.org/10.1038/s41598-019-56785-1
- Stone, E. (2020, September 30). Meet THCP and CBDP: Study reveals the identification of two new cannabinoids. Leafly. https://www.leafly.com/news/science-tech/thcp-cbdp-study-reveals-identification-two-new-cannabinoids
- Awakened Tropicals. (2021, March 16). Meet the Raw Cannabinoids. https://www.awakenedtopicals.com/blog/2019/7/10/meet-the-raw-cannabinoids
- About time. (n.d.-e). Phytocannabinoids vs Endocannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/phytocannabinoids-vs-endocannabinoids-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330635278?prev_url=detail
- About time. (n.d.-g). The Entourage Effect [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-entourage-effect-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330634809?prev_url=detail
- About time. (n.d.-d). Main Benefits of Marijuana Cannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/main-benefits-of-marijuana-cannabinoids-information-poster-with-benefits-of-marijuana-cannabinoids-and-table-of-natural-cannabinoids/414944091?prev_url=detail
- About time. (n.d.-a). Cannabis Terpenes [Graphic]. Adobe Stock. https://stock.adobe.com/images/cannabis-terpenes-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/316181241?prev_url=detail
- Johnson, J., & Theisen, E. (2020, March 6). What to know about terpenes. Medical News Today. https://www.medicalnewstoday.com/articles/what-are-terpenes
- Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., & Jantrawut, P. (2020). The Cannabis Terpenes. Molecules, 25(24), 5792. https://doi.org/10.3390/molecules25245792
- Feelds. (2021, March). Terpenes — Miroboard [Chart]. Miro. https://miro.com/app/board/o9J_ktrdRac=/
- Johnston, G. A. R., Chebib, M., Duke, R. K., Fernandez, S. P., Hanrahan, J. R., Hinton, T., & Mewett, K. N. (2009). Herbal Products and GABA Receptors. Encyclopedia of Neuroscience, 1095–1101. https://doi.org/10.1016/b978-008045046-9.00868-8
- Shiel, W. C. (2017, March 3). Anticoagulant (Blood Thinner) Medical Definition Written by Doctors. MedicineNet. https://www.medicinenet.com/anticoagulant/definition.htm
- Strain Print. (2020, January 24). Understanding Terpenes: Delta 3 Carene. Strainprint Technologies Inc. https://strainprint.ca/understanding-terpenes-delta-3-carene/
- Robbins, C. (2020, September 8). Delta 3 Carene: The Terpene That Promotes Healthy Bones (& Dry Mouth). Cannabis Aficionado. https://cannabisaficionado.com/delta-3-carene/
- Seol, G. H., & Kim, K. Y. (2016). Eucalyptol and Its Role in Chronic Diseases. Advances in Experimental Medicine and Biology, 389–398. https://doi.org/10.1007/978-3-319-41342-6_18
- National Center for Biotechnology Information. (2021c). Geraniol. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Geraniol
- National Center for Biotechnology Information. (2021b). gamma-Linolenic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5280933
- Fan, Y.-Y., & Chapkin, R. S. (1998). Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. The Journal of Nutrition, 128(9), 1411–1414. https://doi.org/10.1093/jn/128.9.1411
- Dictionary.com. (n.d.). Definition of antiproliferative | Dictionary.com. Www.Dictionary.Com. https://www.dictionary.com/browse/antiproliferative
- RxList. (2019, September 17). Gamma Linolenic Acid: Health Benefits, Uses, Side Effects, Dosage & Interactions. https://www.rxlist.com/gamma_linolenic_acid/supplements.htm
- Hartsel, J. A., Eades, J., Hickory, B., & Makriyannis, A. (2016). Cannabis sativa and Hemp. Nutraceuticals, 735–754. https://doi.org/10.1016/b978-0-12-802147-7.00053-x
- Bennett, P. (2020b, July 28). What is humulene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/humulene-terpene
- National Center for Biotechnology Information. (2021d). Limonene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/22311
- Cancer.Net Editorial Board. (2020, October 21). Chemoprevention. Cancer.Net. https://www.cancer.net/navigating-cancer-care/prevention-and-healthy-living/chemoprevention
- National Center for Biotechnology Information. (2021e). Linalool. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/6549
- About time. (n.d.-f). Terpenes in CBD Oil [Graphic]. Adobe Stock. https://stock.adobe.com/images/terpenes-in-cbd-oil-with-structural-formulas-vertical-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/289977909?prev_url=detail
- Gotter, A., & Murrell, D. (2019, March 7). A Guide to Antiseptics. Healthline. https://www.healthline.com/health/what-is-antiseptic
- Tilray. (2020b, July 28). Cannabis Terpenes: The Benefits of Humulene, Caryophyllene, and Trans-Nerolidol. Leafly. https://www.leafly.com/news/cannabis-101/humulene-caryophyllene-and-trans-nerolidol-what-are-the-benefits
- Centers for Disease Control and Prevention. (2020, May 19). CDC – Leishmaniasis – General Information – Frequently Asked Questions (FAQs). https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html
- BD Editors. (2017, June 13). Isomer. Biology Dictionary. https://biologydictionary.net/isomer/
- Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., L.D. Jayaweera, S., A. Dias, D., Sharopov, F., Taheri, Y., Martins, N., Baghalpour, N., C. Cho, W., & Sharifi-Rad, J. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9(11), 738. https://doi.org/10.3390/biom9110738
- Bennett, P. (2020c, July 28). What is ocimene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/benefits-of-ocimene-terpene
- Mayo Clinic. (2021, January 16). High blood pressure (hypertension) – Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/symptoms-causes/syc-20373410
- National Center for Biotechnology Information. (2021a). alpha-Terpinene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/7462
- Barnes, S. (2021, February 1). What is Terpinene | Terpinene Definition by. Weedmaps. https://weedmaps.com/learn/dictionary/terpinene
- Tilray. (2020a, July 28). Benefits of Cannabis Terpenes: Terpineol, Valencene, and Geraniol. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-terpenes-terpineol-valencene-geraniol
- Adlin, B. (2020, July 28). What is terpinolene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/strains-products/least-common-terpene-terpinolene-effects
- Lodi, M. (2019, July 24). Terpenes 411: Valencene. MedMen. https://www.medmen.com/blog/guides/terpenes-411-valencene
- Merriam-Webster Dictionary. (1919). anti-allergic. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/anti-allergic
- Nam, J. H., Nam, D.-Y., & Lee, D.-U. (2016). Valencene from the Rhizomes of Cyperus rotundus Inhibits Skin Photoaging-Related Ion Channels and UV-Induced Melanogenesis in B16F10 Melanoma Cells. Journal of Natural Products, 79(4), 1091–1096. https://doi.org/10.1021/acs.jnatprod.5b01127
- Rea, K. A., Casaretto, J. A., Al-Abdul-Wahid, M. S., Sukumaran, A., Geddes-McAlister, J., Rothstein, S. J., & Akhtar, T. A. (2019). Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry, 164, 162–171. https://doi.org/10.1016/j.phytochem.2019.05.009
- Gardner, F. (n.d.). The Cannflavins Unique to Cannabis | O’Shaughnessy’s. O’Shaughnessy’s Online. https://beyondthc.com/the-cannflavins-unique-to-cannabis/
- Yang, X., Jiang, Y., Yang, J., He, J., Sun, J., Chen, F., Zhang, M., & Yang, B. (2015). Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends in Food Science & Technology, 44(1), 93–104. https://doi.org/10.1016/j.tifs.2015.03.007
- Merriam-Webster Dictionary. (n.d.). Genus. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/genus
- Lipophilicity | Medicinal Chemistry. (n.d.). Omicsonline. https://www.omicsonline.org/lipophilicity-scholarly-open-access-journals.php
- Henderson, R. (2014, November 13). Catatonia and Catalepsy. Patient. https://patient.info/doctor/catatonia-and-catalepsy
- Ricciotti, E., & FitzGerald, G. A. (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(5), 986–1000. https://doi.org/10.1161/atvbaha.110.207449
- WebMD. (n.d.). Indomethacin Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing – WebMD. https://www.webmd.com/drugs/2/drug-8880-5186/indomethacin-oral/indomethacin-oral/details
- Radwan, M. M., ElSohly, M. A., Slade, D., Ahmed, S. A., Wilson, L., El-Alfy, A. T., Khan, I. A., & Ross, S. A. (2008). Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry, 69(14), 2627–2633. https://doi.org/10.1016/j.phytochem.2008.07.010
- FAIRBAIRN, J. W., & PICKENS, J. O. A. N. T. (1979). THE ORAL ACTIVITY OF Δ′-TETRAHYDROCANNABINOL AND ITS DEPENDENCE ON PROSTAGLANDIN E2. British Journal of Pharmacology, 67(3), 379–385. https://doi.org/10.1111/j.1476-5381.1979.tb08691.x
- Barrett, M. L., Gordon, D., & Evans, F. J. (1985). Isolation from cannabis sativa L. of cannflavin—a novel inhibitor of prostaglandin production. Biochemical Pharmacology, 34(11), 2019–2024. https://doi.org/10.1016/0006-2952(85)90325-9
- Werz, O., Seegers, J., Schaible, A. M., Weinigel, C., Barz, D., Koeberle, A., Allegrone, G., Pollastro, F., Zampieri, L., Grassi, G., & Appendino, G. (2014). Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition, 2(3), 53–60. https://doi.org/10.1016/j.phanu.2014.05.001
- Bautista, J. L., Yu, S., & Tian, L. (2021). Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega, 6(8), 5119–5123. https://doi.org/10.1021/acsomega.1c00318
- Salem, M. M., Capers, J., Rito, S., & Werbovetz, K. A. (2011). Antiparasitic Activity of C-Geranyl Flavonoids from Mimulus bigelovii. Phytotherapy Research, 25(8), 1246–1249. https://doi.org/10.1002/ptr.3404
- Huang, E. S., Strate, L. L., Ho, W. W., Lee, S. S., & Chan, A. T. (2011). Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. The American Journal of Medicine, 124(5), 426–433. https://doi.org/10.1016/j.amjmed.2010.12.022
- Erridge, S., Mangal, N., Salazar, O., Pacchetti, B., & Sodergren, M. H. (2020). Cannflavins – From plant to patient: A scoping review. Fitoterapia, 146, 104712. https://doi.org/10.1016/j.fitote.2020.104712
- Iannotti, F. A., de Maio, F., Panza, E., Appendino, G., Taglialatela-Scafati, O., de Petrocellis, L., Amodeo, P., & Vitale, R. M. (2020). Identification and Characterization of Cannabimovone, a Cannabinoid from Cannabis sativa, as a Novel PPARγ Agonist via a Combined Computational and Functional Study. Molecules, 25(5), 1119. https://doi.org/10.3390/molecules25051119
- Carreras, J., Kirillova, M. S., & Echavarren, A. M. (2016). Synthesis of (−)-Cannabimovone and Structural Reassignment of Anhydrocannabimovone through Gold(I)-Catalyzed Cycloisomerization. Angewandte Chemie International Edition, 55(25), 7121–7125. https://doi.org/10.1002/anie.201601834
- National Library of Medicine. (n.d.). cannabimovone – Search Results. PubMed. Retrieved September 9, 2021, from https://pubmed.ncbi.nlm.nih.gov/?term=cannabimovone&sort=date
- Marani, L. (2020, April 1). Introducing CBM (Cannabimovone), the newly-individuated cannabinoid. Leaf Report. https://www.leafreport.com/news/introducing-cbm-cannabimovone-the-newly-individuated-cannabinoid-4356
- Robinson, R. (2020, March 3). What Is CBM and What Does This Uncommon Cannabinoid Do? Merry Jane. https://merryjane.com/culture/what-is-cbm-and-what-does-this-uncommon-cannabinoid-do
- Lewis, A. C. (2021, September 3). Cannabis Researchers Seek to Unlock the Healing Power of Pot. WSJ. https://www.wsj.com/articles/cannabis-researchers-seek-to-unlock-the-healing-power-of-pot-11630674984
- Taglialatela-Scafati, O., Pagani, A., Scala, F., de Petrocellis, L., di Marzo, V., Grassi, G., & Appendino, G. (2010). Cannabimovone, a Cannabinoid with a Rearranged Terpenoid Skeleton from Hemp. European Journal of Organic Chemistry, 2010(11), 2067–2072. https://doi.org/10.1002/ejoc.200901464
- Russo, E. B., & Marcu, J. (2017). Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Cannabinoid Pharmacology, 67–134. https://doi.org/10.1016/bs.apha.2017.03.004
- Wood, T. B., Spivey, W. T. N., & Easterfield, T. H. (1899). III.—Cannabinol. Part I. J. Chem. Soc., Trans., 75(0), 20–36. https://doi.org/10.1039/ct8997500020