“The endocannabinoid system has been found to be pervasive throughout mammalian species. It has also been described in invertebrate species as primitive as the Hydra.” 8
The Endocannabinoid System
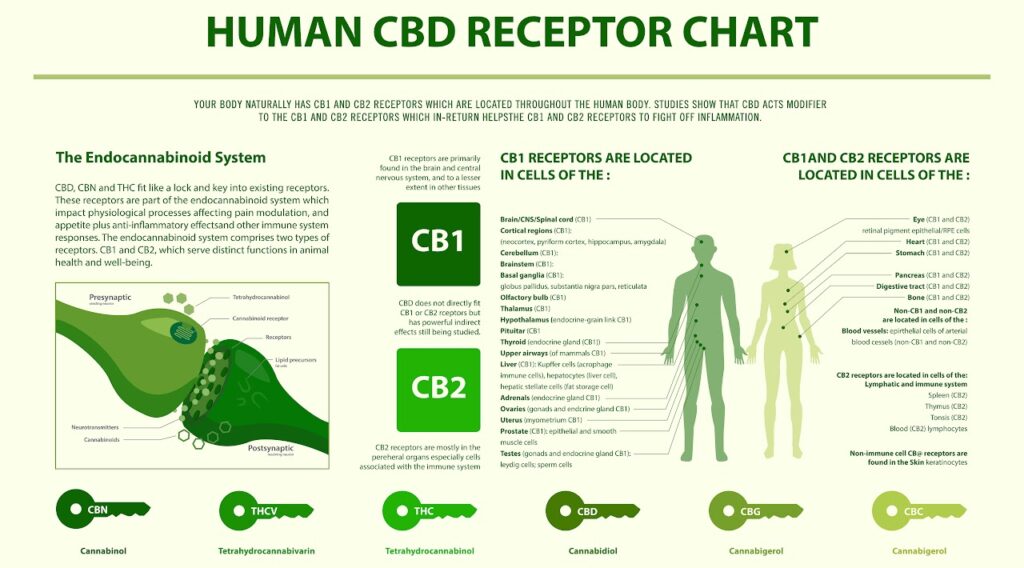
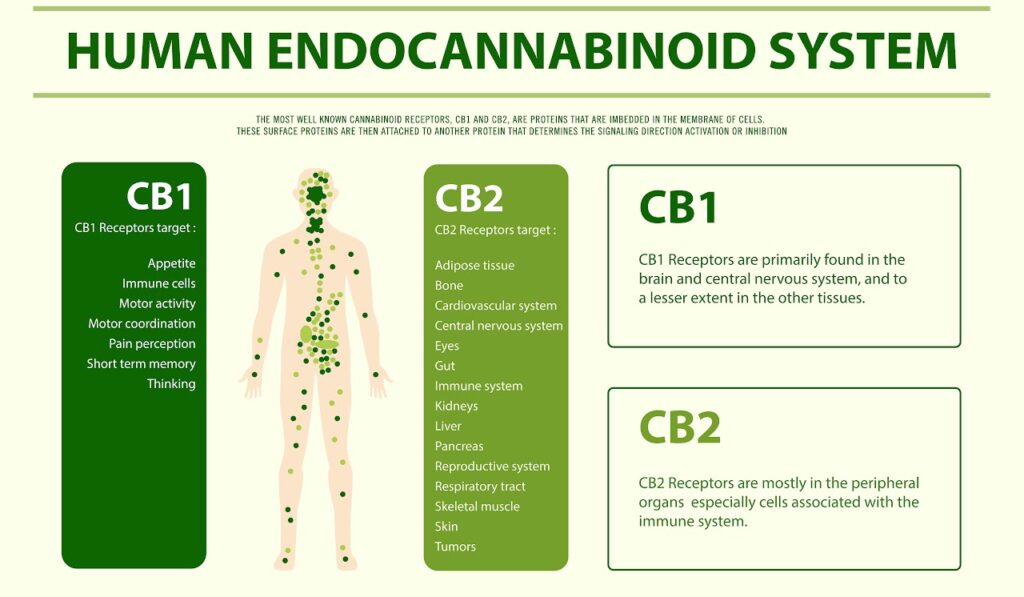
The endocannabinoid system consists of cannabinoid receptor type-1 (CB1R) and cannabinoid receptor type-2 (CB2R)4. Endocannabinoid system research also investigates endogenous ligands (endocannabinoids) — along with the enzymes responsible for metabolizing cannabis4,8.
Conceptions, definitions, and theories about the endocannabinoid system have continued to expand over the last two decades4. Experts predict the definition of “endocannabinoids” is bound to change as research continues to develop, as well.
For a complex flowchart of the endocannabinoid system, please refer to page 2 of this study by Di Marzo and Piscitelli (2015) in the Neurotherapeutics journal.
The endocannabinoid system is involved in regulating a variety of biological processes, including22:
- Brain reward systems
- Drug addiction
- Metabolic processes (such as homeostasis and glucose metabolism37)
- Memory
- Social, behavioral, and emotional responses37.
Evolutionary, it’s believed the endocannabinoid system developed concurrently with the nervous system as multicellular animals evolved8. However, it wasn’t discovered until the mid-1990s.
CB1 & CB2 Receptors
Although the name “endocannabinoid receptors” inadvertently implies all cannabinoids target these receptors, it’s still unclear how CBD and some of its non-psychoactive derivatives interact with the endocannabinoid system2,7. As a Healthline article by Crystal Raypole & Alan Carter, Pharm.D. explains:
“Experts aren’t completely sure how CBD interacts with the ECS. But they do know that it doesn’t bind to CB1 or CB2 receptors the way THC does.
Instead, many believe it works by preventing endocannabinoids from being broken down. This allows them to have more of an effect on your body. Others believe that CBD binds to a receptor that hasn’t been discovered yet.”
Alternatively, a 2015 Neurotherapeutics Journal study suggests that cannabinoid receptors be renamed “THC/THCV” receptors since THC and THCV are the only cannabinoids that bind with high-affinity to CB1 and CB24.
Moreover, the authors propose the definition of “cannabinoid receptors” should be expanded to include proteins that are modulated by cannabinoids, such as the transient receptor potential (TRP) channels.
Naturally, the authors then advocate for expanding the definition of “endocannabinoid enzymes” to include additional enzymes that mediate the endocannabinoid system. They also note that other researchers sometimes use an extended definition of the endocannabinoid system called the “enlarged endocannabinoid system.”
Transient Receptor Potential (TRP) Channels
Transient Receptor Potential (TRP) channels are a group of cell membrane (the membrane is the outermost layer of a cell) proteins involved in the transduction of chemical and electric stimuli9. TRPs mediate neural signals involved in sensing temperature, pressure, pH, smell, taste, vision, and pain perception by modulating ion entry.
“Many diseases involve TRP channel dysfunction, including neuropathic pain, inflammation, and respiratory diseases,” says a study by Muller et al9. “In the pursuit of new treatments for these disorders, it was discovered that cannabinoids can modulate a certain subset of TRP channels.”
Furthermore, all cannabinoids can influence these channels — that includes endogenous, phytogenic, and synthetic cannabinoids.
There are a total of six TRP subfamilies recognized in mammals. The three TRP subfamilies that cannabinoids can modulate include:
- TRP vanilloid (TRPV)
- TRP ankyrin (TRPA)
- TRP melastatin (TRPM)
Of these three subfamilies, six TRP channels in particulate are modulated by cannabinoids:
- TRPV1
- TRPV2
- TRPV3
- TRPV4
- TRPA1
- TRPM8
(For context, there are 28 different TRP channels across all six TRP subfamilies.)
These six TRP channels mediated by cannabinoids are sometimes referred to as “ionotropic cannabinoid receptors”9,13.
TRPV1 and at least five other TRP channels have been discovered in the dorsal root ganglia, which carry sensory signals from the peripheral nervous system (the nerves and ganglion that branch out from the brain and spinal cord) to the central nervous system (the brain and spinal cord)9,10.
“Until recently, the dorsal root ganglion has been considered a passive organ that metabolically assists functions and pathways between the PNS and CNS. New studies suggest, however, that the DRG is an active participant in peripheral processes, including PAF injury, inflammation, and neuropathic pain development.”10
Desensitization, particularly through TRPV1, is a key feature currently being explored9. TRPV1 becomes rapidly desensitized when activated, which produces analgesic (pain relieving) effects.
Hence, understanding the interaction between cannabinoids and transient receptor potential channels (TRPs) could provide valuable insight into the cause of cannabis’ pain-alleviating effects.
So far, it appears CB1 receptors colocalize (occur within the same cell) with TRP channels in brain and sensory neurons. On the other hand, CB2 receptors colocalize in sensory neurons and osteoclasts (osteoclasts are cells responsible for the dissolution and absorption of bone12).
Anandamide (an endocannabinoid) activates TRPV1 and blocks TRPM8. N-arachidonyl dopamine (another endocannabinoid) blocks TRPM8, too.
THC “acts most potently at TRPV2” and “moderately modulates” TRPV3, TRPV4, TRPA1, and TRPM89. However, it does not appear to modulate TRPV1 — the TRP channel researchers are exploring for its analgesic effects.
On the other hand, CBD (which has anti-inflammatory properties14,15), shows “little affinity” for CB1 and CB2 receptors, yet CBD has the most potent effects on TRPV1 and TRPM89. CBD also activates TRPV2, TRPA1, and TRPV314.
Additionally, the synthetic cannabinoid “WIN55,212-2” exerts analgesic effects by desensitizing TRPV1 and TRPA1, further supporting the notion that TRPV1 plays a central role in the perception of neuropathic pain and other “harmful stimuli”16.
“CBD not only activates TRP through a direct agonist-receptor interaction, but also by lowering the level of oxidative stress.”14
Key Takeaways
The Endocannabinoid system primarily consists of CB1 and CB2 receptors, as well as endocannabinoids (cannabinoids naturally produced by your body, regardless of cannabis consumption)4,8. However, many have proposed the endocannabinoid system should be expanded to include other receptors, metabolites, and proteins, as THC is the only psychoactive cannabinoid that targets CB1 and CB2 receptors.
ECS receptors are located throughout your body and regulate a variety of biological processes, including your brain’s reward systems, metabolic processes, and memory22,37. The ECS also regulates addiction, social, behavioral, and emotional responses.
Both phytocannabinoids and endocannabinoids have extensive influence over TRP channels, which serve a variety of functions throughout the body, as well9. As a result, some experts refer to TRP channels as “ionotropic cannabinoid receptors”9,13.
Although it’s widely believed that the ECS evolved alongside the nervous system in mammals, it wasn’t discovered until the mid-1990s.
Thus, despite the well-recognized interplay between cannabinoids, the endocannabinoid system, terpenes, and flavonoids, many areas of cannabis pharmacokinetics remain critically under-researched.
Nevertheless, our understanding of the ECS is rapidly evolving as research progresses in the field of cannabis research.
References
- Spindle, T. R., Bonn-Miller, M. O., & Vandrey, R. (2019). Changing landscape of cannabis: novel products, formulations, and methods of administration. Current Opinion in Psychology, 30, 98–102. https://doi.org/10.1016/j.copsyc.2019.04.002
- Morales, P., Hurst, D. P., & Reggio, P. H. (2017). Molecular Targets of the Phytocannabinoids: A Complex Picture. Progress in the Chemistry of Organic Natural Products, 103–131. https://doi.org/10.1007/978-3-319-45541-9_4
- WeedMaps. (n.d.). What is a Phytocannabinoid? | Phytocannabinoid Definition by Weedmaps. https://weedmaps.com/learn/dictionary/phytocannabinoid/
- Di Marzo, V., & Piscitelli, F. (2015). The Endocannabinoid System and its Modulation by Phytocannabinoids. Neurotherapeutics, 12(4), 692–698. https://doi.org/10.1007/s13311-015-0374-6
- About time. (n.d.-b). Human CBD Receptor Chart [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-cbd-receptor-chart-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/267901368?asset_id=267901368
- About time. (n.d.-c). Human Endocannabinoid System [Infographic]. Adobe Stock. https://stock.adobe.com/images/human-endocannabinoid-system-endocannabinoid-system-horizontal-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/276860924?prev_url=detail
- Raypole, C., & Carter, A. (2019, May 17). A Simple Guide to the Endocannabinoid System. Healthline. https://www.healthline.com/health/endocannabinoid-system
- Silver, R. J. (2019). The Endocannabinoid System of Animals. Animals, 9(9), 686. https://doi.org/10.3390/ani9090686
- Muller, C., Morales, P., & Reggio, P. H. (2019). Cannabinoid Ligands Targeting TRP Channels. Frontiers in Molecular Neuroscience, 11, 0. https://doi.org/10.3389/fnmol.2018.00487
- Ahimsadasan, N. (2021, January 13). Neuroanatomy, Dorsal Root Ganglion – StatPearls – NCBI Bookshelf. NCBI. https://www.ncbi.nlm.nih.gov/books/NBK532291/
- marina_ua. (n.d.). The nervous system [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-nervous-system/94711659?prev_url=detail
- The Editors of Encyclopaedia Britannica. (2014, January). Osteoclast | cell. Encyclopedia Britannica. https://www.britannica.com/science/osteoclast
- Akopian, A. N., Ruparel, N. B., Jeske, N. A., Patwardhan, A., & Hargreaves, K. M. (2009). Role of ionotropic cannabinoid receptors in peripheral antinociception and antihyperalgesia. Trends in Pharmacological Sciences, 30(2), 79–84. https://doi.org/10.1016/j.tips.2008.10.008
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants, 9(1), 21. https://doi.org/10.3390/antiox9010021
- Pellati, F., Borgonetti, V., Brighenti, V., Biagi, M., Benvenuti, S., & Corsi, L. (2018). Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Research International, 2018, 1–15. https://doi.org/10.1155/2018/1691428
- Marrone, M. C., Morabito, A., Giustizieri, M., Chiurchiù, V., Leuti, A., Mattioli, M., Marinelli, S., Riganti, L., Lombardi, M., Murana, E., Totaro, A., Piomelli, D., Ragozzino, D., Oddi, S., Maccarrone, M., Verderio, C., & Marinelli, S. (2017). TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nature Communications, 8(1), 0. https://doi.org/10.1038/ncomms15292
- Centers For Disease Control and Prevention. (2017, August 21). About synthetic cannabinoids. https://www.cdc.gov/nceh/hsb/chemicals/sc/About.html
- Wiley, J., Marusich, J., Huffman, J. W., Balster, R. L., & Thomas, B. (2011). Hijacking of Basic Research: The Case of Synthetic Cannabinoids. RTI Press, 0. https://doi.org/10.3768/rtipress.2011.op.0007.1111
- Peñaloza, M. (2018, July 27). NPR Cookie Consent and Choices. NPR. https://choice.npr.org/index.html?origin=https://www.npr.org/2018/07/27/632261920/d-c-has-had-more-than-300-suspected-k2-overdoses-in-2-weeks
- Maccarrone, M. (2020). Phytocannabinoids and endocannabinoids: different in nature. Rendiconti Lincei. Scienze Fisiche e Naturali, 31(4), 931–938. https://doi.org/10.1007/s12210-020-00957-z
- Rahn, B. (2020, September 29). The entourage effect: How cannabis compounds may be working together. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-entourage-effect-why-thc-and-cbd-only-medicines-arent-g
- Gülck, T., & Møller, B. L. (2020). Phytocannabinoids: Origins and Biosynthesis. Trends in Plant Science, 25(10), 985–1004. https://doi.org/10.1016/j.tplants.2020.05.005
- Beadle, A. (2020, July 27). CBG vs CBD: What Are the Differences? Analytical Cannabis. https://www.analyticalcannabis.com/articles/cbg-vs-cbd-what-are-the-differences-312232
- Bennett, P. (2020a, July 28). THCA and CBD Crystalline: Cannabinoids at Their Purest. Leafly. https://www.leafly.com/news/strains-products/what-are-thca-cbda-crystalline-cannabinoids
- Morano, A., Fanella, M., Albini, M., Cifelli, P., Palma, E., Giallonardo, A. T., & Di Bonaventura, C. (2020). Cannabinoids in the Treatment of Epilepsy: Current Status and Future Prospects. Neuropsychiatric Disease and Treatment, Volume 16, 381–396. https://doi.org/10.2147/ndt.s203782
- Scaccia, A., & Wilson, D. (2020, August 19). Serotonin: What You Need to Know. Healthline. https://www.healthline.com/health/mental-health/serotonin
- Ameri, A. (1999). The effects of cannabinoids on the brain. Progress in Neurobiology, 58(4), 315–348. https://doi.org/10.1016/s0301-0082(98)00087-2
- Lucas, C. J., Galettis, P., & Schneider, J. (2018, July 12). The pharmacokinetics and the pharmacodynamics of cannabinoids. British Journal of Clinical Pharmacology. https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bcp.13710
- National Cancer Institute. (n.d.). NCI Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/delta-8-tetrahydrocannabinol?redirect=true
- Shannon, S. (2019). Cannabidiol in Anxiety and Sleep: A Large Case Series. The Permanente Journal, 23, 0. https://doi.org/10.7812/tpp/18-041
- Grandy, D. (2016). Amphetamines Activate G-Protein Coupled Trace Amine-Associated Receptor 1 (TAAR1). In Neuropathology of drug addictions and substance misuse. (pp. 108–116). Academic Press,. https://doi.org/10.1016/B978-0-12-800212-4.00010-8
- Raypole, C., & Sullivan, D. (2020, March 30). Cannabis Got You Paranoid? How to Deal With It. Healthline. https://www.healthline.com/health/marijuana-paranoia
- Walters, O., & Kerr, M. (2020, October 7). A Straightforward Cannabinoid Chart With Simple Explanations. Sovereignty. https://sovereignty.co/cannabinoid-chart/
- S. (2020, July 15). The Terpenes Of Cannabis, Their Aromas, And Effects. THCFarmer – Cannabis Cultivation Network. https://www.thcfarmer.com/threads/the-terpenes-of-cannabis-their-aromas-and-effects.68222/
- Marijuana Doctors. (2018, November 20). Cannabivarin (CBV) | What Is CBV. https://www.marijuanadoctors.com/resources/cannabinoids/cannabivarin-cbv/
- Pretzsch, C. M., Voinescu, B., Lythgoe, D., Horder, J., Mendez, M. A., Wichers, R., Ajram, L., Ivin, G., Heasman, M., Edden, R. A. E., Williams, S., Murphy, D. G. M., Daly, E., & McAlonan, G. M. (2019). Effects of cannabidivarin (CBDV) on brain excitation and inhibition systems in adults with and without Autism Spectrum Disorder (ASD): a single dose trial during magnetic resonance spectroscopy. Translational Psychiatry, 9(1), 0. https://doi.org/10.1038/s41398-019-0654-8
- Abioye, A., Ayodele, O., Marinkovic, A., Patidar, R., Akinwekomi, A., & Sanyaolu, A. (2020). Δ9-Tetrahydrocannabivarin (THCV): a commentary on potential therapeutic benefit for the management of obesity and diabetes. Journal of Cannabis Research, 2(1), 0. https://doi.org/10.1186/s42238-020-0016-7
- García, C., Palomo-Garo, C., García-Arencibia, M., Ramos, J. A., Pertwee, R. G., & Fernández-Ruiz, J. (2011). Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. British Journal of Pharmacology, 163(7), 1495–1506. https://doi.org/10.1111/j.1476-5381.2011.01278.x
- Seladi-Schulman, J., & Kay, C. (2019, August 19). In Vivo vs. In Vitro: What Does It All Mean? Healthline. https://www.healthline.com/health/in-vivo-vs-in-vitro
- Coombs, D. W. & The Journal of the American Medical Association. (1983, November 4). antinociception. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/medical/antinociception
- Citti, C., Linciano, P., Russo, F., Luongo, L., Iannotta, M., Maione, S., Laganà, A., Capriotti, A. L., Forni, F., Vandelli, M. A., Gigli, G., & Cannazza, G. (2019). A novel phytocannabinoid isolated from Cannabis sativa L. with an in vivo cannabimimetic activity higher than Δ9-tetrahydrocannabinol: Δ9-Tetrahydrocannabiphorol. Scientific Reports, 9(1), 0. https://doi.org/10.1038/s41598-019-56785-1
- Stone, E. (2020, September 30). Meet THCP and CBDP: Study reveals the identification of two new cannabinoids. Leafly. https://www.leafly.com/news/science-tech/thcp-cbdp-study-reveals-identification-two-new-cannabinoids
- Awakened Tropicals. (2021, March 16). Meet the Raw Cannabinoids. https://www.awakenedtopicals.com/blog/2019/7/10/meet-the-raw-cannabinoids
- About time. (n.d.-e). Phytocannabinoids vs Endocannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/phytocannabinoids-vs-endocannabinoids-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330635278?prev_url=detail
- About time. (n.d.-g). The Entourage Effect [Graphic]. Adobe Stock. https://stock.adobe.com/images/the-entourage-effect-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/330634809?prev_url=detail
- About time. (n.d.-d). Main Benefits of Marijuana Cannabinoids [Graphic]. Adobe Stock. https://stock.adobe.com/images/main-benefits-of-marijuana-cannabinoids-information-poster-with-benefits-of-marijuana-cannabinoids-and-table-of-natural-cannabinoids/414944091?prev_url=detail
- About time. (n.d.-a). Cannabis Terpenes [Graphic]. Adobe Stock. https://stock.adobe.com/images/cannabis-terpenes-horizontal-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/316181241?prev_url=detail
- Johnson, J., & Theisen, E. (2020, March 6). What to know about terpenes. Medical News Today. https://www.medicalnewstoday.com/articles/what-are-terpenes
- Sommano, S. R., Chittasupho, C., Ruksiriwanich, W., & Jantrawut, P. (2020). The Cannabis Terpenes. Molecules, 25(24), 5792. https://doi.org/10.3390/molecules25245792
- Feelds. (2021, March). Terpenes — Miroboard [Chart]. Miro. https://miro.com/app/board/o9J_ktrdRac=/
- Johnston, G. A. R., Chebib, M., Duke, R. K., Fernandez, S. P., Hanrahan, J. R., Hinton, T., & Mewett, K. N. (2009). Herbal Products and GABA Receptors. Encyclopedia of Neuroscience, 1095–1101. https://doi.org/10.1016/b978-008045046-9.00868-8
- Shiel, W. C. (2017, March 3). Anticoagulant (Blood Thinner) Medical Definition Written by Doctors. MedicineNet. https://www.medicinenet.com/anticoagulant/definition.htm
- Strain Print. (2020, January 24). Understanding Terpenes: Delta 3 Carene. Strainprint Technologies Inc. https://strainprint.ca/understanding-terpenes-delta-3-carene/
- Robbins, C. (2020, September 8). Delta 3 Carene: The Terpene That Promotes Healthy Bones (& Dry Mouth). Cannabis Aficionado. https://cannabisaficionado.com/delta-3-carene/
- Seol, G. H., & Kim, K. Y. (2016). Eucalyptol and Its Role in Chronic Diseases. Advances in Experimental Medicine and Biology, 389–398. https://doi.org/10.1007/978-3-319-41342-6_18
- National Center for Biotechnology Information. (2021c). Geraniol. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Geraniol
- National Center for Biotechnology Information. (2021b). gamma-Linolenic acid. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/5280933
- Fan, Y.-Y., & Chapkin, R. S. (1998). Importance of Dietary γ-Linolenic Acid in Human Health and Nutrition. The Journal of Nutrition, 128(9), 1411–1414. https://doi.org/10.1093/jn/128.9.1411
- Dictionary.com. (n.d.). Definition of antiproliferative | Dictionary.com. Www.Dictionary.Com. https://www.dictionary.com/browse/antiproliferative
- RxList. (2019, September 17). Gamma Linolenic Acid: Health Benefits, Uses, Side Effects, Dosage & Interactions. https://www.rxlist.com/gamma_linolenic_acid/supplements.htm
- Hartsel, J. A., Eades, J., Hickory, B., & Makriyannis, A. (2016). Cannabis sativa and Hemp. Nutraceuticals, 735–754. https://doi.org/10.1016/b978-0-12-802147-7.00053-x
- Bennett, P. (2020b, July 28). What is humulene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/humulene-terpene
- National Center for Biotechnology Information. (2021d). Limonene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/22311
- Cancer.Net Editorial Board. (2020, October 21). Chemoprevention. Cancer.Net. https://www.cancer.net/navigating-cancer-care/prevention-and-healthy-living/chemoprevention
- National Center for Biotechnology Information. (2021e). Linalool. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/6549
- About time. (n.d.-f). Terpenes in CBD Oil [Graphic]. Adobe Stock. https://stock.adobe.com/images/terpenes-in-cbd-oil-with-structural-formulas-vertical-business-infographic-illustration-about-cannabis-as-herbal-alternative-medicine-and-chemical-therapy-healthcare-and-medical-science-vector/289977909?prev_url=detail
- Gotter, A., & Murrell, D. (2019, March 7). A Guide to Antiseptics. Healthline. https://www.healthline.com/health/what-is-antiseptic
- Tilray. (2020b, July 28). Cannabis Terpenes: The Benefits of Humulene, Caryophyllene, and Trans-Nerolidol. Leafly. https://www.leafly.com/news/cannabis-101/humulene-caryophyllene-and-trans-nerolidol-what-are-the-benefits
- Centers for Disease Control and Prevention. (2020, May 19). CDC – Leishmaniasis – General Information – Frequently Asked Questions (FAQs). https://www.cdc.gov/parasites/leishmaniasis/gen_info/faqs.html
- BD Editors. (2017, June 13). Isomer. Biology Dictionary. https://biologydictionary.net/isomer/
- Salehi, B., Upadhyay, S., Erdogan Orhan, I., Kumar Jugran, A., L.D. Jayaweera, S., A. Dias, D., Sharopov, F., Taheri, Y., Martins, N., Baghalpour, N., C. Cho, W., & Sharifi-Rad, J. (2019). Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules, 9(11), 738. https://doi.org/10.3390/biom9110738
- Bennett, P. (2020c, July 28). What is ocimene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/science-tech/benefits-of-ocimene-terpene
- Mayo Clinic. (2021, January 16). High blood pressure (hypertension) – Symptoms and causes. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/symptoms-causes/syc-20373410
- National Center for Biotechnology Information. (2021a). alpha-Terpinene. PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/7462
- Barnes, S. (2021, February 1). What is Terpinene | Terpinene Definition by. Weedmaps. https://weedmaps.com/learn/dictionary/terpinene
- Tilray. (2020a, July 28). Benefits of Cannabis Terpenes: Terpineol, Valencene, and Geraniol. Leafly. https://www.leafly.com/news/cannabis-101/cannabis-terpenes-terpineol-valencene-geraniol
- Adlin, B. (2020, July 28). What is terpinolene and what does this cannabis terpene do? Leafly. https://www.leafly.com/news/strains-products/least-common-terpene-terpinolene-effects
- Lodi, M. (2019, July 24). Terpenes 411: Valencene. MedMen. https://www.medmen.com/blog/guides/terpenes-411-valencene
- Merriam-Webster Dictionary. (1919). anti-allergic. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/anti-allergic
- Nam, J. H., Nam, D.-Y., & Lee, D.-U. (2016). Valencene from the Rhizomes of Cyperus rotundus Inhibits Skin Photoaging-Related Ion Channels and UV-Induced Melanogenesis in B16F10 Melanoma Cells. Journal of Natural Products, 79(4), 1091–1096. https://doi.org/10.1021/acs.jnatprod.5b01127
- Rea, K. A., Casaretto, J. A., Al-Abdul-Wahid, M. S., Sukumaran, A., Geddes-McAlister, J., Rothstein, S. J., & Akhtar, T. A. (2019). Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry, 164, 162–171. https://doi.org/10.1016/j.phytochem.2019.05.009
- Gardner, F. (n.d.). The Cannflavins Unique to Cannabis | O’Shaughnessy’s. O’Shaughnessy’s Online. https://beyondthc.com/the-cannflavins-unique-to-cannabis/
- Yang, X., Jiang, Y., Yang, J., He, J., Sun, J., Chen, F., Zhang, M., & Yang, B. (2015). Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends in Food Science & Technology, 44(1), 93–104. https://doi.org/10.1016/j.tifs.2015.03.007
- Merriam-Webster Dictionary. (n.d.). Genus. The Merriam-Webster.Com Dictionary. https://www.merriam-webster.com/dictionary/genus
- Lipophilicity | Medicinal Chemistry. (n.d.). Omicsonline. https://www.omicsonline.org/lipophilicity-scholarly-open-access-journals.php
- Henderson, R. (2014, November 13). Catatonia and Catalepsy. Patient. https://patient.info/doctor/catatonia-and-catalepsy
- Ricciotti, E., & FitzGerald, G. A. (2011). Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 31(5), 986–1000. https://doi.org/10.1161/atvbaha.110.207449
- WebMD. (n.d.). Indomethacin Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing – WebMD. https://www.webmd.com/drugs/2/drug-8880-5186/indomethacin-oral/indomethacin-oral/details
- Radwan, M. M., ElSohly, M. A., Slade, D., Ahmed, S. A., Wilson, L., El-Alfy, A. T., Khan, I. A., & Ross, S. A. (2008). Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry, 69(14), 2627–2633. https://doi.org/10.1016/j.phytochem.2008.07.010
- FAIRBAIRN, J. W., & PICKENS, J. O. A. N. T. (1979). THE ORAL ACTIVITY OF Δ′-TETRAHYDROCANNABINOL AND ITS DEPENDENCE ON PROSTAGLANDIN E2. British Journal of Pharmacology, 67(3), 379–385. https://doi.org/10.1111/j.1476-5381.1979.tb08691.x
- Barrett, M. L., Gordon, D., & Evans, F. J. (1985). Isolation from cannabis sativa L. of cannflavin—a novel inhibitor of prostaglandin production. Biochemical Pharmacology, 34(11), 2019–2024. https://doi.org/10.1016/0006-2952(85)90325-9
- Werz, O., Seegers, J., Schaible, A. M., Weinigel, C., Barz, D., Koeberle, A., Allegrone, G., Pollastro, F., Zampieri, L., Grassi, G., & Appendino, G. (2014). Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition, 2(3), 53–60. https://doi.org/10.1016/j.phanu.2014.05.001
- Bautista, J. L., Yu, S., & Tian, L. (2021). Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega, 6(8), 5119–5123. https://doi.org/10.1021/acsomega.1c00318
- Salem, M. M., Capers, J., Rito, S., & Werbovetz, K. A. (2011). Antiparasitic Activity of C-Geranyl Flavonoids from Mimulus bigelovii. Phytotherapy Research, 25(8), 1246–1249. https://doi.org/10.1002/ptr.3404
- Huang, E. S., Strate, L. L., Ho, W. W., Lee, S. S., & Chan, A. T. (2011). Long-Term Use of Aspirin and the Risk of Gastrointestinal Bleeding. The American Journal of Medicine, 124(5), 426–433. https://doi.org/10.1016/j.amjmed.2010.12.022
- Erridge, S., Mangal, N., Salazar, O., Pacchetti, B., & Sodergren, M. H. (2020). Cannflavins – From plant to patient: A scoping review. Fitoterapia, 146, 104712. https://doi.org/10.1016/j.fitote.2020.104712